Knockdown vs Knockout: Efficacy and Safety of RNAi
- Lauren Wilkes
- May 14, 2025
- 3 min read
Authored by: Lauren Wilkes
Art by: Mia Hsu
So much of the discussion for how we approach curing genetic diseases centers around how we genetically “engineer,” edit, induce changes, etc in DNA to reverse or correct mutations. As the concept of gene editing and genetic engineering gains traction , terms like “CRISPR” have become adjacent to a biology buzz word. However, the conversations behind the scenes are much more complex. The reality of considerations about gene editing and gene therapy center around efficiency, efficacy, and – crucially – the safety of gene-altering mechanisms. One emerging, yet still under-discussed gene editing approach that has piqued the interest of many for its safety implications, is RNA Interference (RNAi). RNA Interference is a process through which a specific mRNA is degraded via targeting with an RNA sequence. With gene therapy through gene editing being as prominent a conversation as it has become, safety is naturally a concern. Fortunately, research published by the American Journal of Transplantation and the NIH indicates that RNAi is a promising alternative that can minimize some safety risks with gene modification.
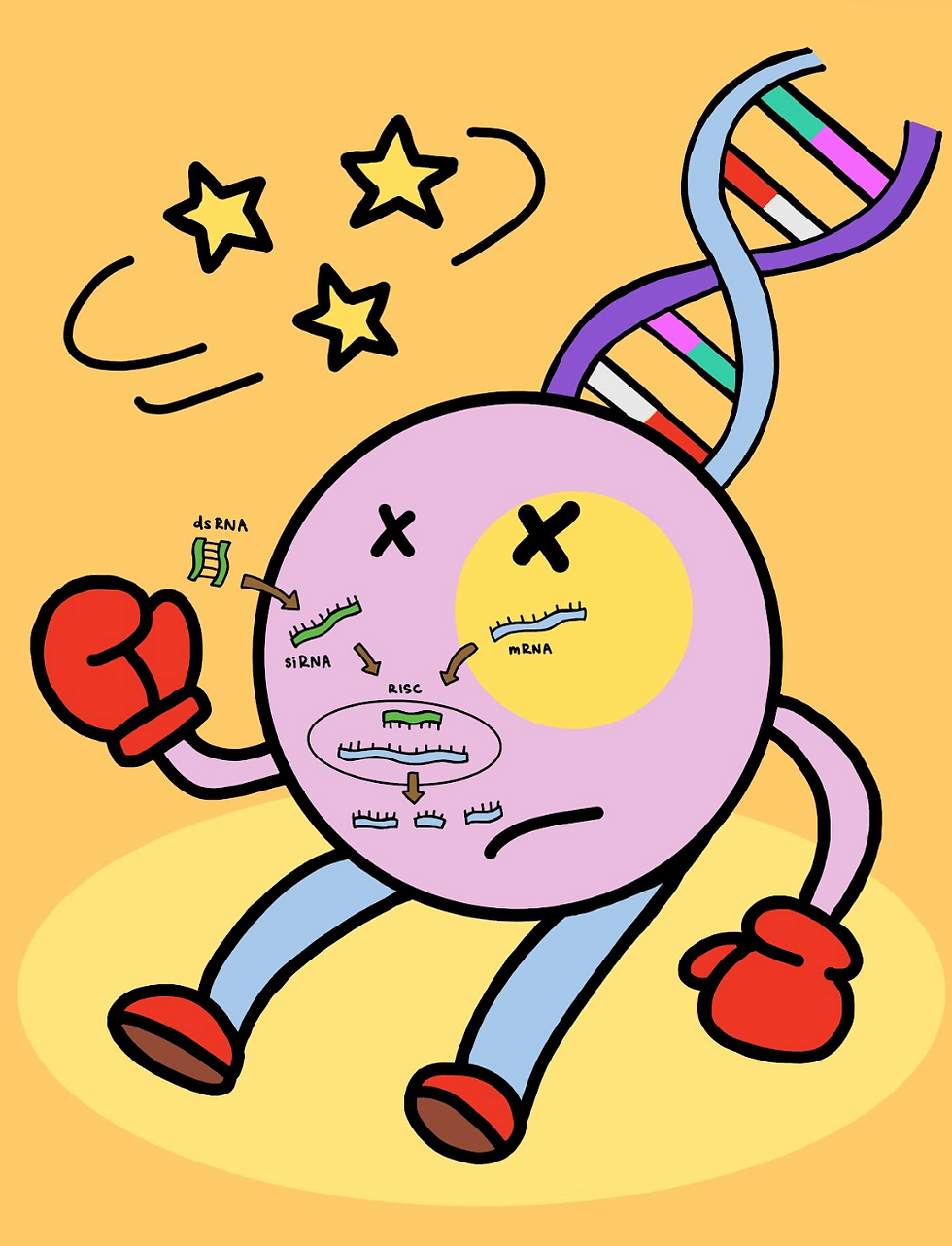
RNAi operates through the use of shorter, non-coding RNA molecules. In other words, through RNA molecules that don’t implicitly encode a protein, and therefore have no specified function [2]. These small interfering RNA molecules are more commonly referred to as siRNAs. SiRNAs are integral to RNAi’s gene “silencing” ability. The mechanism surrounding siRNAs follows a few steps. First, siRNAs are initially formed via an enzyme colloquially termed “Dicer,” immediately after which they are bound within a complex known as the RNA-induced Silencing Complex (RISC). The siRNAs are then able to successfully guide the RISC to the targeted mRNA strand and bind resulting in either degradation or repression of the subsequent translation that would otherwise take place. As a result, gene expression that would otherwise emanate from the translation is “silenced” [3].
So, what makes RNAi any safer than other gene editing tools such as CRISPR? In essence, RNAi is actually a reversible mechanism. Due to the fact that no edits are actually made to the DNA transcript (i.e. solely to the mRNA that will be translated), none of the changes are permanent or heritable. In contrast, the CRISPR complex makes a double-stranded break directly into the DNA sequence, creating irreversible mutations. Unlike such irrevertible “knockout” mutations,, RNAi, rather, induces “knockdown” mutations in which gene expression/function is knocked down until such time as it is reverted [4]. The transient knockdown nature of RNAi allows researchers to study gene function without experiencing the irreversible consequences of permanent gene damage. Additionally, RNA’s lack of impact on the DNA sequence itself prevents it from being inherited, unlike CRISPR’s direct DNA alterations. These characteristics of RNAi make its efficacy and increased safety over other genetic engineering mechanisms quite clear.
It is important to note that, like any effective gene editing tool, RNAi has its own shortcomings. One concern researchers raise is with potential “off-target” effects of RNAi due to the siRNAs ability to bind to other potential complementary mRNA strands [5]. Despite this, RNAi has been used safely and effectively in recent years. To name a few, in 2018, the APOLLO trial was completed which resulted in the RNAi therapeutic being FDA approved [6] and in 2022, investigational clinical studies of RNAi in lung, CNS, and skeletal muscle targets were conducted [7]. The many facets of RNAi that drastically increase its safety, efficacy, and efficiency over other irreversible and heritable gene silencing mechanisms, such as the CRISPR-Cas9 system, make it a promising option for advancing gene therapy.
References:
Ichim, T. E., Li, M., Qian, H., Popov, I. A., Rycerz, K., Zheng, X., White, D., Zhong, R., & Min, W. P. (2004). RNA interference: a potent tool for gene-specific therapeutics. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 4(8), 1227–1236. https://doi.org/10.1111/j.1600-6143.2004.00530.x
Mattick, J. S., & Makunin, I. V. (2006). Non-coding RNA. Human Molecular Genetics, 15(suppl_1), R17–R29. https://doi.org/10.1093/hmg/ddl046
Dana, H., Chalbatani, G. M., Mahmoodzadeh, H., Karimloo, R., Rezaiean, O., Moradzadeh, A., Mehmandoost, N., Moazzen, F., Mazraeh, A., Marmari, V., Ebrahimi, M., Rashno, M. M., Abadi, S. J., & Gharagouzlo, E. (2017). Molecular Mechanisms and Biological Functions of siRNA. International journal of biomedical science : IJBS, 13(2), 48–57. https://pmc.ncbi.nlm.nih.gov/articles/PMC5542916/
Prabhune, M. (2019). Synthego | Full Stack Genome Engineering. Synthego.com. https://www.synthego.com/blog/rnai-vs-crispr-guide
Boettcher, M., & McManus, M. T. (2015). Choosing the Right Tool for the Job: RNAi, TALEN, or CRISPR. Molecular cell, 58(4), 575–585. https://doi.org/10.1016/j.molcel.2015.04.028
Alnylam Announces First-Ever FDA Approval of an RNAi Therapeutic, ONPATTROTM (patisiran) for the Treatment of. (n.d.). Investor Relations | Alnylam Pharmaceuticals, Inc. https://investors.alnylam.com/press-release?id=22946
Zhang, M. M., Bahal, R., Rasmussen, T. P., Manautou, J. E., & Zhong, X. B. (2021). The growth of siRNA-based therapeutics: Updated clinical studies. Biochemical pharmacology, 189, 114432. https://doi.org/10.1016/j.bcp.2021.114432






'Knockdown vs Knockout,' huh? This RNAi stuff sounds kinda neat, especially how it's reversible compared to CRISPR. I was just reading about similar genetic engineering techniques on my commute this morning, so it's interesting to see RNAi approved by FDA in 2018! What's next?YouTube Playlist Length Calculator